
A study of 21,848 non-emergent, high-risk patients who underwent percutaneous coronary intervention (PCI) with percutaneous ventricular assist devices (PVADs), including Impella, demonstrates the PVAD patients had a lower rate of mortality and complications than patients who underwent PCI with intra-aortic balloon pumps (IABPs). The study, by Al-khadra, et al., published in the February 15, 2020 print edition of Catheterization & Cardiovascular Interventions.
In the analysis, the PVAD cohort was significantly sicker than the IABP cohort. PVAD patients were older and had higher rates of hypertension, diabetes, hyperlipidemia, prior PCI, prior coronary artery bypass graft surgery, anemia, chronic lung disease, liver disease, renal failure, and peripheral vascular disease.
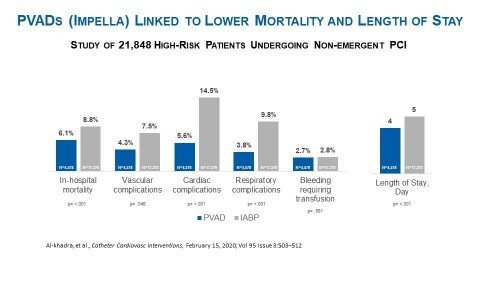
As detailed in figure 1, despite the higher rates of comorbidities, when investigators used multivariate logistic regression (n=21,848) they determined, compared to the IABP patients, PVAD patients were associated with:
- Lower in-hospital mortality (6.1% vs. 8.8%, p= <.001)
- Lower vascular complications (4.3% vs. 7.5%, p=.046)
- Lower cardiac complications (5.6% vs. 14.5%, p= <.001)
- Lower respiratory complications (3.8% vs. 9.8%, p= <.001)
- A similar rate of bleeding to IABP patients (2.7% vs. 2.8%, p=.581)
Furthermore, propensity score matching (n=1,926) demonstrated that, compared to the IABP patients, PVAD patients had:
- Lower in-hospital mortality (3.5% vs. 6.4%, p= <.012)
- Lower vascular complications (3.4% vs. 6.0%, p=.017)
- Lower cardiac complications (3.4% vs. 12.2%, p= <.001)
- Lower respiratory complications (2.6% vs. 6.1%, p=.001)
- A similar rate of bleeding to IABP patients (2.6% vs. 2.4%, p=.795)
Investigators obtained their data from the National Inpatient Sampling (NIS) database, the largest all-payer inpatient care database in the United States. NIS is developed through a partnership with the Agency for Healthcare Research and Quality (AHRQ) and contains data on approximately eight million Medicare and private payer hospital stays. The authors disclosed and described in detail the methods for the study population, patient and hospital characteristics and statistical analysis, including linear regression models and propensity score matching.
“This analysis is another example of real-world data demonstrating improved outcomes for patients and reduced length of stay when PVADs are used,” said Perwaiz Meraj, MD, one of the study’s authors and director of interventional cardiology at Zucker School of Medicine at Hofstra/Northwell, Northwell Health in Manhasset, NY. “The use of best practices, techniques and technologies can enable safer, more complete revascularization that improves patient outcomes and quality of life.”
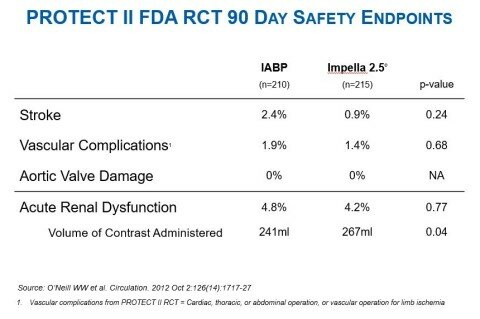
The Al-khadra, et al. publication also noted that PVAD patients had a shorter length of stay than IABP patients (4 days vs. 5 days p= <.001). This finding is consistent with the PROTECT II randomized controlled trial (see figure 2) and multiple other peer-reviewed studies, including the Maini, et al. publication in Expert Review of Pharmacoeconomics & Outcomes Research. Maini et al. appraises the findings and conclusions of six publications and found PVADs, specifically Impella 2.5, are associated with reduced hospital length of stay and are cost-effective when compared with IABP.
“High-risk PCI patients often pose a challenge to the interventionalist due to patient comorbidities which drive worse outcomes,” said Cindy Grines, MD, a study author and an interventional cardiologist and chief scientific officer at Northside Hospital Cardiovascular Institute in Atlanta. “This publication demonstrates the rationale for PVAD use during high-risk PCI. Left ventricular support maintains coronary perfusion during periods of transient hypotension during long or repeated inflations necessary to achieve complete revascularization.”
The Al-khadra et al. study was conducted independently by physician-investigators and not funded or reviewed by Abiomed. Study sites are Cleveland Clinic, Detroit Medical Center, Beth Israel Deaconess Medical Center/ Harvard Medical School, MedStar Washington Hospital Center, St. John Hospital and Medical Center, Emory University, Zucker School of Medicine at Hofstra Northwell Health, Henry Ford Health System, Keele University and Royal Stoke University Hospital.
The Impella heart pump is manufactured by Abiomed (NASDAQ: ABMD) and is the most studied mechanical circulatory support device in the history of the FDA with more than 14 years of FDA studies, real world clinical data on more than 140,000 patients, and more than 650 peer-reviewed publications.












